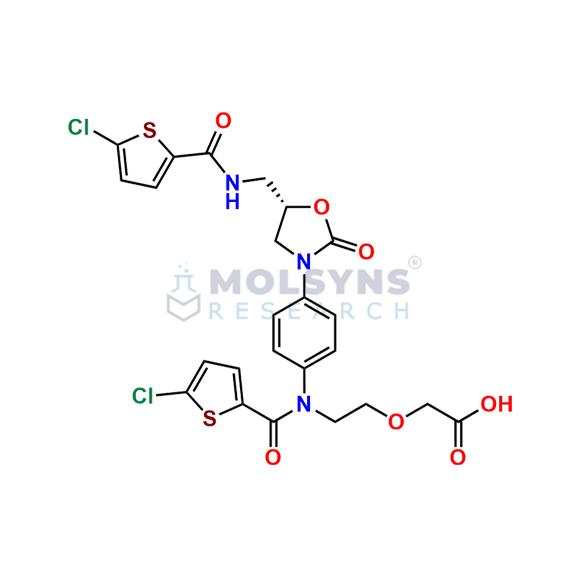
Rivaroxaban EP Impurity I
Product Type
Impurity Standards
CAT. No.
MRL-R08009
CAS. No.
1151893-81-0
Mol. F.
C24H21Cl2N3O7S2
Mol. Wt.
598.48
Synonyms
Rivaroxaban N-acyl glycolic acid (USP)
Chemical Name
[2-[15,95-dichloro-2,52,8-trioxo-3,7-diaza-5(3,5)-1,3-oxazolidina-1,9(2)-dithiophena-4(1,4)-benzenanonaphan-3-yl]ethoxy]acetic acid (as per EP)
Stock Status
In Stock
Rivaroxaban EP Impurity I with CAS No: 1151893-81-0 is an impurity standard of Rivaroxaban. Rivaroxaban is a medication classified as a direct oral anticoagulant (DOAC) or novel oral anticoagulant (NOAC).Rivaroxaban is used to prevent and treat blood clots, particularly in conditions such as deep vein thrombosis (DVT), pulmonary embolism (PE), and atrial fibrillation. Rivaroxaban helps to reduce the risk of excessive blood clotting by inhibiting the activity of certain clotting factors in the blood. Buy high quality Rivaroxaban EP Impurity I from Molsyns Research. Molsyns Research is one of the leading manufacturer and exporter of Rivaroxaban EP Impurity I
