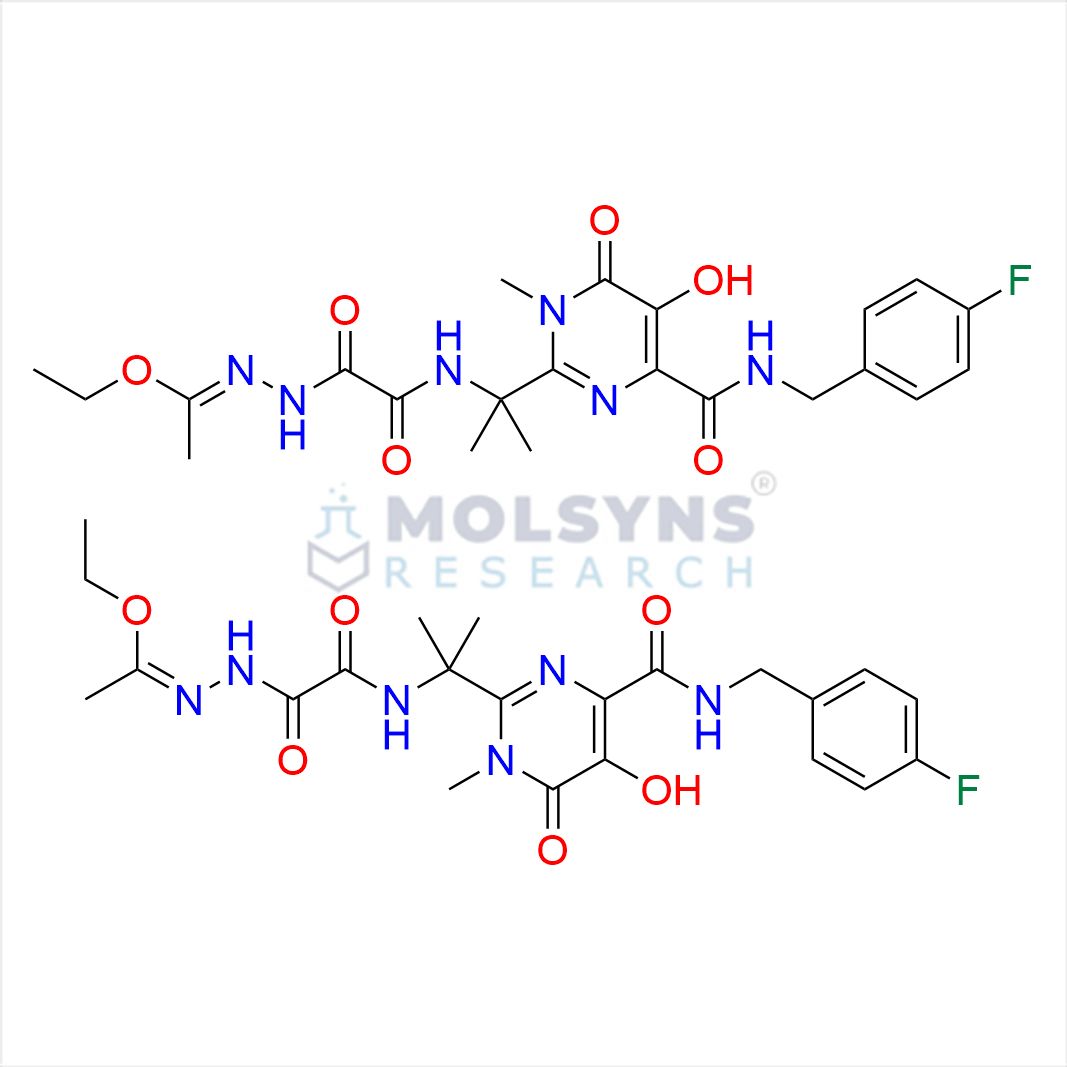
Raltegravir EP Impurity G & F Mixture
Product Type
Impurity Standards
CAT. No.
MRL-R52008
CAS. No.
NA
Mol. F.
C44H54F2N12O12
Mol. Wt.
980.98
Synonyms
NA
Chemical Name
Ethyl (E)-N-(2-((2-(4-((4-fluorobenzyl)carbamoyl)-5-hydroxy-1-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)propan-2-yl)amino)-2-oxoacetyl)acetohydrazonate compound with ethyl (Z)-N-(2-((2-(4-((4-fluorobenzyl)carbamoyl)-5-hydroxy-1-methyl-6-oxo-1,6-dihydropyrimi
Stock Status
Custom Synthesis
Raltegravir EP Impurity G & F Mixture is an impurity standard of Raltegravir. Raltegravir is a medication used to treat HIV infection. It belongs to a class of drugs called integrase inhibitors, which work by blocking the action of the enzyme integrase, preventing the integration of the HIV virus into the DNA of human immune cells. By doing so, raltegravir helps reduce the replication of the virus and slows the progression of HIV infection. Buy high quality Raltegravir EP Impurity G & F Mixture from Molsyns Research. Molsyns Research is one of the leading manufacturer and exporter of Raltegravir EP Impurity G & F Mixture
Request for Quotation : Raltegravir EP Impurity G & F Mixture
Related Products
Raltegravir
Raltegravir EP impurity A
Raltegravir EP Impurity B
Raltegravir EP Impurity C
Raltegravir EP Impurity D
Raltegravir EP Impurity E
Raltegravir EP Impurity H
Raltegravir Diketo Ethoxy Impurity
Raltegravir Diketo Methoxy Impurity
Raltegravir Impurity 2
Raltegravir Diketo Amine Impurity
Raltegravir Impurity 10
