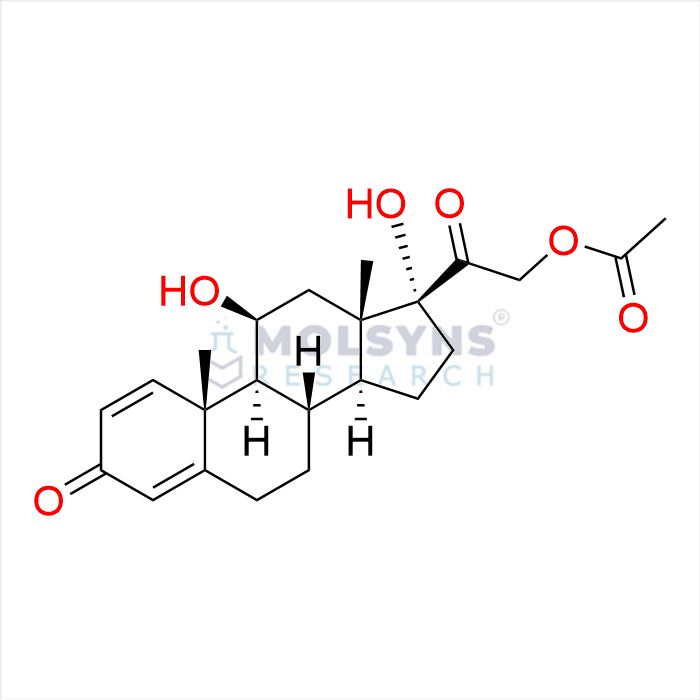
Hydrocortisone Acetate EP Impurity C
Product Type
Impurity Standards
CAT. No.
MRL-H09004
CAS. No.
52-21-1
Mol. F.
C23H30O6
Mol. Wt.
402.49
Synonyms
prednisolone acetate
Chemical Name
11β,17-Dihydroxy-3,20-dioxopregna-1,4-dien-21-yl acetate
Stock Status
Custom Synthesis
Hydrocortisone Acetate EP Impurity C with CAS No: 52-21-1 is an impurity standard of Hydrocortisone. Hydrocortisone is a corticosteroid medication widely used for its anti-inflammatory and immunosuppressive properties. Its application extends to the treatment of various skin conditions, including eczema, dermatitis, and insect bites. Chemically, hydrocortisone is a synthetic form of cortisol, a natural hormone produced by the adrenal glands. By inhibiting the release of inflammatory substances and suppressing immune responses, hydrocortisone helps alleviate symptoms such as redness, itching, and swelling. Topical formulations of hydrocortisone are commonly available over-the-counter, providing relief for mild to moderate skin irritations. However, prolonged or inappropriate use of corticosteroids may lead to side effects, emphasizing the importance of careful application and medical guidance. Buy high quality Hydrocortisone Acetate EP Impurity C from Molsyns Research. Molsyns Research is one of the leading manufacturer and exporter of Hydrocortisone Acetate EP Impurity C
Request for Quotation : Hydrocortisone Acetate EP Impurity C
Related Products
Hydrocortisone
Hydrocortisone EP Impurity A
Hydrocortisone EP Impurity B
Hydrocortisone EP Impurity D
Hydrocortisone EP Impurity E
Hydrocortisone EP Impurity F
Hydrocortisone EP Impurity G
Hydrocortisone EP Impurity H
Hydrocortisone EP Impurity I
Hydrocortisone EP Impurity J
Hydrocortisone EP Impurity M
Hydrocortisone EP Impurity N
Hydrocortisone EP Impurity O
Hydrocortisone Butyrate
Hydrocortisone EP Impurity C
Hydrocortisone 17,21-Methyl Orthobutyrate
Hydrocortisone-21-Butyrate
Hydrocortisone Impurity B
Hydrocortisone 17-Succinate
Hydrocortisone-21-Valerate
Hydrocortisone Acetate EP Impurity E
Hydrocortisone Acetate EP Impurity F
Hydrocortisone Acetate EP Impurity G
Hydrocortisone Glyoxal Hydrate Isomer-I
Hydrocortisone Glyoxal Hydrate Isomer-II
17-Keto Hydroxyprogesterone
Hydrocortisone Oxo Acetic Acid
Hydrocortisone Impurity F
Hydrocortisone Impurity G
Hydrocortisone Impurity H
Hydrocortisone Impurity 1
Hydrocortisone Impurity 2
Hydrocortisone Impurity 3
Hydrocortisone Valerate Impurity 3
9α-Bromohydrocortisone Acetate
Δ9(11)-Hydrocortisone 17-Valerate
Hydrocortisone Un-Symmetrical Dimer
Cortisone hemisuccinate
Hydrocortisone Hemisuccinate
Delta 6 Hydrocortisone Hemisuccinate
Hydrocortisone Cortienyl Aldehyde Impurity
Hydrocortisone Impurity 4
Hydrocortisone Impurity 6
9 Alpha Hydroxyl Hydrocortisone Acetate
Hydrocortisone 17-Butyrate 3-Enol Methyl Ether
Hydrocortisone Impurity 7
Hydrocortisone Impurity 8
Hydrocortisone Impurity 9
Hydrocortisone Impurity 10
17-Dehydro-21-Hydroxy Hydrocortisone
Hydrocortisone Impurity 12
Hydrocortisone Impurity 13
21-Dehydrocortisone
Androst-4-ene-17-carboxylic acid
Hydrocortisone Impurity 16
Hydrocortisone Impurity 17
Hydrocortisone Impurity 18
Hydrocortisone Impurity 19
Hydrocortisone Impurity D
Hydrocortisone Impurity 21
Hydrocortisone 17-phosphate
6-Beta Hydroxy Hydrocortisone Phosphate
Hydrocortisone Impurity 23
Hydrocortisone Impurity 24
Hydrocortisone 21-phosphate
Hydrocortisone 21-Carboxylic Acid
Hydrocortisone Impurity 25
Epi Hydrocortisone Hemisuccinate
21-Dehydrocorticosterone
Hydrocortisone (9β,11β)-Epoxide
Delta 6,7 Cortisone Acetate
Delta 8,9 Cortisone Acetate
6α-Hydroxy-11-Deoxycortisol
3-Enol Ether Cortisone Acetate
Hydrocortisone Oxo Acetic Acid (Z-Isomer)
Hydrocortisone Oxo Acetic Acid (E-Isomer)
Hydrocortisone Z-enol aldehyde
