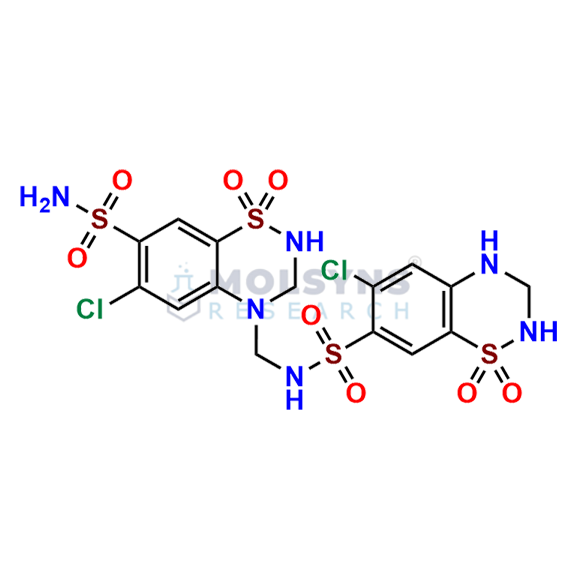
Hydrochlorothiazide EP impurity C
Product Type
Impurity Standards
CAT. No.
MRL-H06004
CAS. No.
402824-96-8
Mol. F.
C15H16Cl2N6O8S4
Mol. Wt.
607.47
Synonyms
Hydrochlorothiazide Dimer
Chemical Name
6-chloro-N-[(6-chloro-7-sulfamoyl-2,3-dihydro-4H-1,2,4-benzothiadiazin-4-yl 1,1-dioxide)methyl]-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide
Stock Status
In Stock
Hydrochlorothiazide EP Impurity C is a impurity of hydrochlorothiazide, a medication employed to treat high blood pressure and edema. Impurity C is categorized as a pharmaceutical reference standard, specifically associated with hydrochlorothiazide. Its principal role is in pharmaceutical quality control, serving as a reference standard for precise analytical testing. This testing enables the accurate identification and quantification of Impurity C within hydrochlorothiazide-based medications, ensuring their purity and compliance with stringent regulatory standards. Buy high quality Hydrochlorothiazide EP impurity C from Molsyns Research. Chemecia Pharma is one of the leading manufacturer and exporter of Hydrochlorothiazide EP impurity C
